Environmental Impacts on Enzyme Activity: Understanding Temperature, pH, and Other Factors
Introduction: The Crucial Role of Enzyme Activity in Biological Systems
Enzymes are specialized proteins that accelerate chemical reactions in living organisms, making them essential for processes ranging from digestion to DNA replication. However, enzymes are not immune to changes in their surroundings. The environment in which an enzyme operates-particularly its temperature, pH, and the concentrations of various molecules-plays a critical role in determining how effectively it can perform its function. This article explores how environmental conditions can affect enzymes, what specifically happens when factors like temperature or pH shift, and actionable guidance for optimizing enzyme activity in lab, industrial, or educational settings.
How Environmental Factors Affect Enzyme Activity
Environmental factors influence enzyme activity by altering the enzyme’s structure, stability, and the rate at which it catalyzes reactions. The most significant environmental variables include:
- Temperature
- pH (acidity or alkalinity)
- Enzyme and substrate concentrations
- Salt concentration
- Presence of inhibitors or activators
- Water availability (especially in soil and environmental contexts)
Each enzyme is optimized to function efficiently within a narrow range of these conditions. Deviations from this range can lead to reduced activity or even irreversible changes to the enzyme’s structure and function [2] .
Temperature: Balancing Efficiency and Denaturation
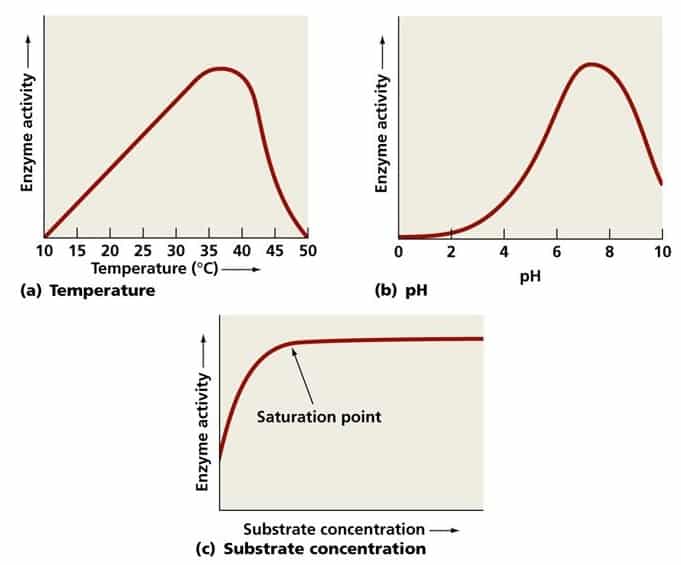
Temperature is one of the most impactful variables affecting enzyme activity. As the temperature increases, so does the kinetic energy of molecules, which typically leads to a higher rate of enzymatic reactions. This is because more frequent collisions occur between enzyme and substrate molecules, increasing the likelihood of reactions taking place [2] .
However, this beneficial effect has a limit. Once the temperature surpasses the enzyme’s optimum, the increased energy begins to disrupt the bonds holding the enzyme’s three-dimensional structure together. This process, known as
denaturation
, leads to a loss of shape and, critically, of catalytic ability. At this point, the enzyme can no longer bind to its substrate or facilitate the reaction efficiently, and the process may become irreversible
[3]
.
Conversely, temperatures lower than the optimal decrease molecular motion, resulting in fewer collisions and a slower reaction rate. The enzyme remains functional but works much less efficiently. In natural settings, such as soils, enzymes can adapt to higher temperatures or periods of dryness, indicating some flexibility depending on environmental context [1] .

Source: studymarxianism.z21.web.core.windows.net
Key Takeaway: Always aim to conduct enzymatic reactions at the enzyme’s optimal temperature. For most human enzymes, this is around 37°C (98.6°F), but this value can differ significantly for enzymes from other organisms or environmental samples.
pH: The Impact of Acidity and Alkalinity
pH is a measure of how acidic or basic a solution is, and it can dramatically affect enzyme structure and function. Each enzyme has an optimal pH that supports its unique three-dimensional structure. Deviations from this optimal pH can alter the charge properties of the amino acids that make up the enzyme, disrupting ionic and hydrogen bonds that are critical for maintaining its shape [2] .
Denaturation may also occur if the pH is extremely high or low, rendering the enzyme inactive. For example, pepsin, a digestive enzyme in the stomach, operates best in acidic conditions (pH 1.5-2), whereas trypsin, found in the small intestine, requires a more alkaline environment (pH 7.5-8.5). Even slight shifts in pH away from these values will substantially reduce enzymatic activity [4] .
Practical Guidance: When working with enzymes, always test and adjust the pH of your reaction mixture to match the enzyme’s known optimum. Use buffer solutions to maintain a stable pH during experiments or industrial processes.
Other Environmental Changes That Affect Enzyme Activity
Enzyme and Substrate Concentrations
The concentrations of enzyme and substrate directly influence the rate of the reaction. Generally, increasing the concentration of either component will increase the reaction rate-up to a point. When all enzyme molecules are engaged with substrate, the reaction reaches a maximum speed and adding more substrate has no further effect [2] .
Implementation Tip: In laboratory and industrial settings, titrate substrate and enzyme concentrations to identify the point of saturation, ensuring cost-effective and efficient use of resources.
Salt Concentration
Salt ions influence the ionic bonds that stabilize enzyme structure. Most enzymes have an optimal salt concentration, and deviations can cause denaturation or altered activity. In high-salt environments, such as marine systems, enzymes may be specially adapted to remain stable and functional.
Practical Application: Use caution when adding salts or buffers to reaction mixtures, and consult enzyme datasheets for compatibility information.
Presence of Inhibitors and Activators
Inhibitors are molecules that decrease enzyme activity, while activators enhance it. These can be naturally occurring or intentionally added in research and industry. Competitive inhibitors bind to the enzyme’s active site, blocking substrate access, while non-competitive inhibitors change enzyme shape by binding elsewhere. Activators may induce a conformational change that increases activity.
Real-World Example: Many drugs are enzyme inhibitors-statins, for example, inhibit HMG-CoA reductase to lower cholesterol.
Water Availability (Especially in Soils)
For enzymes in environmental samples like soil, water content is a crucial factor. Both excess and deficiency can impair enzyme activity. Recent research shows that soil microbial communities can adapt to function optimally under high temperatures and low water availability, particularly in arid and semi-arid environments. These adaptations are important for ecosystem-level processes such as carbon cycling [1] .
Guidance for Researchers: When measuring enzyme activity in environmental samples, document temperature and water content carefully, and replicate field conditions as closely as possible in the lab.
What Happens When an Enzyme’s Environment Changes?
When temperature, pH, or other environmental factors shift away from optimal values, several outcomes are possible:
- Decreased activity : The enzyme may still function but at a reduced rate.
- Denaturation : Irreversible loss of structure and function, particularly at extreme temperatures or pH levels.
- Altered specificity : In rare cases, significant changes can cause the enzyme to bind different substrates or catalyze unintended reactions.
- Adaptation : Over time, especially in environmental contexts, enzyme systems may evolve greater flexibility or stability.
For example, a sudden rise in temperature during an industrial fermentation process can destroy the enzymes responsible for product synthesis, leading to batch failure. In research, ignoring optimal pH can result in irreproducible or misleading results.
Step-by-Step Guidance for Optimizing Enzyme Activity
- Determine the enzyme’s optimal conditions : Consult scientific literature, manufacturer datasheets, or conduct pilot experiments to find the optimal temperature, pH, and salt concentration.
- Prepare reaction buffers : Use appropriate buffer solutions to maintain pH and ionic strength throughout the experiment.
- Control temperature accurately : Use water baths, incubators, or temperature-controlled reactors to keep reactions within the optimal range.
- Monitor concentrations : Adjust enzyme and substrate concentrations to identify the most efficient reaction setup without unnecessary waste.
- Document and replicate environmental conditions : Especially for field or environmental samples, record temperature, pH, water content, and any other relevant parameters.
- Be aware of inhibitors or activators : Screen reaction mixtures for substances that may unintentionally inhibit or activate your enzyme of interest.
- Consult with experts or manufacturers : If unusual results occur, contact the enzyme supplier or consult with experienced colleagues for troubleshooting.
For more detailed instructions or troubleshooting, you can contact enzyme manufacturers directly or consult standard laboratory manuals. When working with environmental or microbial enzymes, consider collaborating with specialists in environmental microbiology or soil science for best practices.
Alternative Approaches and Solutions
When an enzyme proves unstable under your required conditions, consider the following alternatives:
- Enzyme engineering : Modify the enzyme’s amino acid sequence to increase stability.
- Immobilization : Attach enzymes to solid supports to enhance stability and allow reuse.
- Use of extremophilic enzymes : Source enzymes from organisms adapted to extreme environments (thermophiles, acidophiles, etc.) for challenging industrial processes.
- Chemical modifications : Add stabilizing agents or co-factors as recommended by published protocols or suppliers.
These strategies are commonly used in the biotechnology and pharmaceutical industries to maintain enzyme activity under harsh process conditions.

Source: alamy.com
Key Takeaways
- Environmental factors such as temperature, pH, and water availability are critical for enzyme activity.
- Outside their optimal conditions, enzymes may show reduced activity, denaturation, or altered specificity.
- Practical optimization requires careful control and documentation of reaction conditions.
- For challenging applications, alternative methods such as enzyme engineering and immobilization may be required.
- For guidance, consult scientific literature, manufacturer datasheets, or reach out to specialists in enzymology or environmental science.
References
- [1] National Library of Medicine (2020). Environmental factors affect the response of microbial extracellular enzyme activity in soils.
- [2] LibreTexts Biology (2022). Factors Affecting Enzyme Activity.
- [3] Catalysis Blog. How Do Environmental Conditions Affect Enzyme Activity?
- [4] Varsity Tutors (2025). Understand the effect of environment of the enzyme.
- [5] Worthington Biochemical. Factors Affecting Enzyme Activity.






